Hello and welcome to the sixth copy of the newsletter!
In my TherapyLive talk I pitched that when we treat radicular pain we should think in terms of nerve health and sensitivity rather than nerve compression (although the former includes the latter!). To illustrate what I mean by "nerve health" I showed pictures from a study by Albrecht et al. in which the authors image neuroinflammation in people with chronic radicular pain. The first picture is cool but not unexpected: the green arrow points to a small patch of yellow in the intervertebral foramen which represents neuroinflammation (or, more accurately, a marker of neuroinflammation).

We have known for some decades that lumbar disc herniations are pro-inflammatory to the surrounding tissues and that this probably drives a lot of acute "radiculitis". In fact, Albrecht was a bit surprised that the signs of neuroinflammation in the foramen were not stronger and more consistent. Maybe it's because the participants in his study had chronic, not acute pain.
The second image is less intuitive: run your eyes down the aggregated images of the spinal columns of the control patients and the patients with radicular pain and you will see that there is more of a yellow hue to the spinal cords of patients with pain.

Although these patients have (or have had) an insult to the nerve roots in their lower lumbar spine, they also have neuroinflammation in their spinal cords, in the lower thoracic and upper lumbar spine. The neuroinflammation has "spread". In cross section, it is more clear.

Whenever I show this image a few people will message me afterwards saying how striking it is. So there is something important here that I want to "zoom in on". And my main stumbling block is, what on earth is neuroinflammation?
This week’s podcast
This week's podcast is an interview with the lead author of the study, Daniel Albrecht (pron. Albright). Dan speaks really well on this subject. In the podcast, he describes the basics of neuroinflammation and the findings of this study. He also takes on the trickier questions that surround this topic and makes it clear what we know and what we don't know. It’s on all major platforms if you search for “The Sciatica Podcast”, or you can click through from this email. Do listen!
Okay, what on earth is neuroinflammation?
Neuroinflammation is not necessarily pathological
The first thing to say about neuroinflammation is that it is not in itself pathological. Just like inflammation in the muscles, joints and skin, inflammation in the nerves arises in response to dangerous stuff like pathogens or tissue damage. Many different cells and molecules work together to limit the danger and repair any harm done. Neuroinflammation only becomes a clinical problem when there is too much of it, or it goes on too long.
Neuroinflammation is a continuum
The second thing to say is that it is not really something that is turned "on" or "off". We use words like "activated" to describe microglia, which makes this confusing. But really it's a "more" or "less" process, not "on" or "off".
At one end of the continuum, transient, low level immune signalling is involved in the normal development of memory and learning and arguably doesn’t qualify as “neuroinflammation” at all. At the other end "very rapid and dramatic" neuroinflammation kicks in after serious nerve injuries.
What’s involved?
Now let's introduce the "characters" of neuroinflammation to tell the story in stages. To me, one of the most challenging aspects of understanding neuroinflammation (to the extent that I have) was wading through the many and various cells and molecules which all seem to do subtle variations of the same thing. For clarity's sake, I have cut out a lot of these (sorry, fans of TLR-4) so that I can tell a coherent "story" within the scope of an email newsletter...
Glia
Glia comes from the Greek word for "glue" because for a long time, it was thought that that's all these cells are. It is only since the 1990s that scientists have begun to appreciate that they have an important, active role in how the nervous system works. The two important types of glia for us are microglia and astroglia.
Microglia
Microglia are the first to respond to a nerve injury. They reside in the CNS and they look like this (like octopuses).
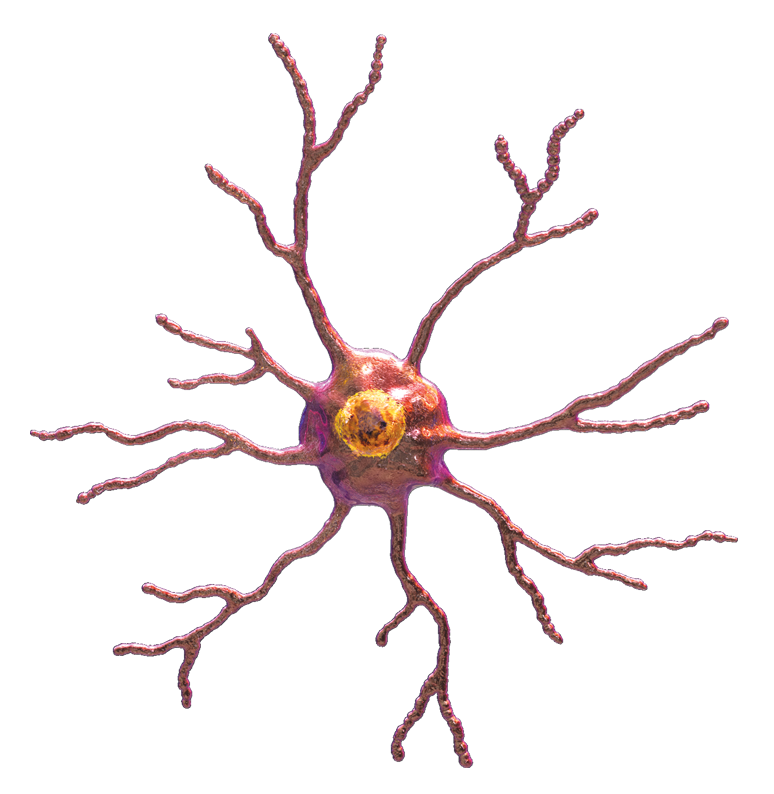
They are not connected to each other. They each have their own domain. Their octopus legs are constantly "feeling" their environment, looking for threats. They support neurons, tweaking their synapses, keeping them healthy and clearing away any debris.

Microglia are "activated" by anything that might mean danger. This could be a nerve injury, including a peripheral nerve injury, but it could also just be a lot of nociception. That said, they are much more reactive to nerve injuries than, say, peripheral inflammation. When activated, microglia pull in their octopus arms and increase in size so that they look like "fat, angry rainclouds"

They express different surface proteins, ready to get to work. And they proliferate and move to the site of danger and other related parts of the spinal cord and brain.
Again, microglia activation is not an accident or a pathology, it is an adaptive response to danger. They are "looking for" danger all the time. And, so they don't miss it, neurons release signalling molecules to "tell them" to activate.

How does this lead to pain?
Activated microglia express proteins that are responsible for releasing inflammatory cytokines (signalling molecules). If you are anything like me, this is where your eyes might start to glaze over. It just becomes a list of things you have no mental image for: TNF-a, IL-1B, BDNF...
I think the fear is that if you forget for a second what, say IL-1B does to GABA then you will miss some vital piece of the puzzle. Luckily, according to the researcher Ru Rong Ji, although the bits and bobs involved in neuroinflammation are diverse, they "modulate pain in surprisingly consistent ways". Essentially, Ji says, cells like microglia "release neuromodulatory substances [the TNF-a, IL-1B, BDNF etc. etc.] in close proximity to nociceptors which either promote or dampen pain depending on the specific identity of the mediators involved". If the balance of these neuromodulatory substances is in favour of those that are pain-promoting, then neuroinflammation is more likely to contribute to pain. (We will come to the pain-dampening ones later...)
Astrocytes
This frenzy of microglia activation is usually short lived. It is probably more involved in acute pain. But, microglia will signal to astrocytes which are slower to activate but more persistent. (This is partly why neuroinflammation is often considered a factor in chronic pain states, while classical inflammation is not.)
Astrocytes look like this.

Like stars, as their name suggests. They are connected to each other, in a fine, fibrous network. This network enwraps synapses (as many as one million per astrocyte) and connects with blood vessels. When activated, astrocytes change their normal, run-of-the-mill communications with neurons and instead tell them to produce more neurotransmitters and inflammatory cytokines.

Immune cells
Astrocytes also contribute to another important feature of neuroinflammation: the blood-nerve barrier, including the blood-brain and blood-spinal cord barriers, becomes more permeable. (It seems that nociception also contributes to this change.) This allows immune cells like macrophages (in the PNS) and t-lymphocytes to more readily enter the neuron itself. Here’s a T-lymphocyte.
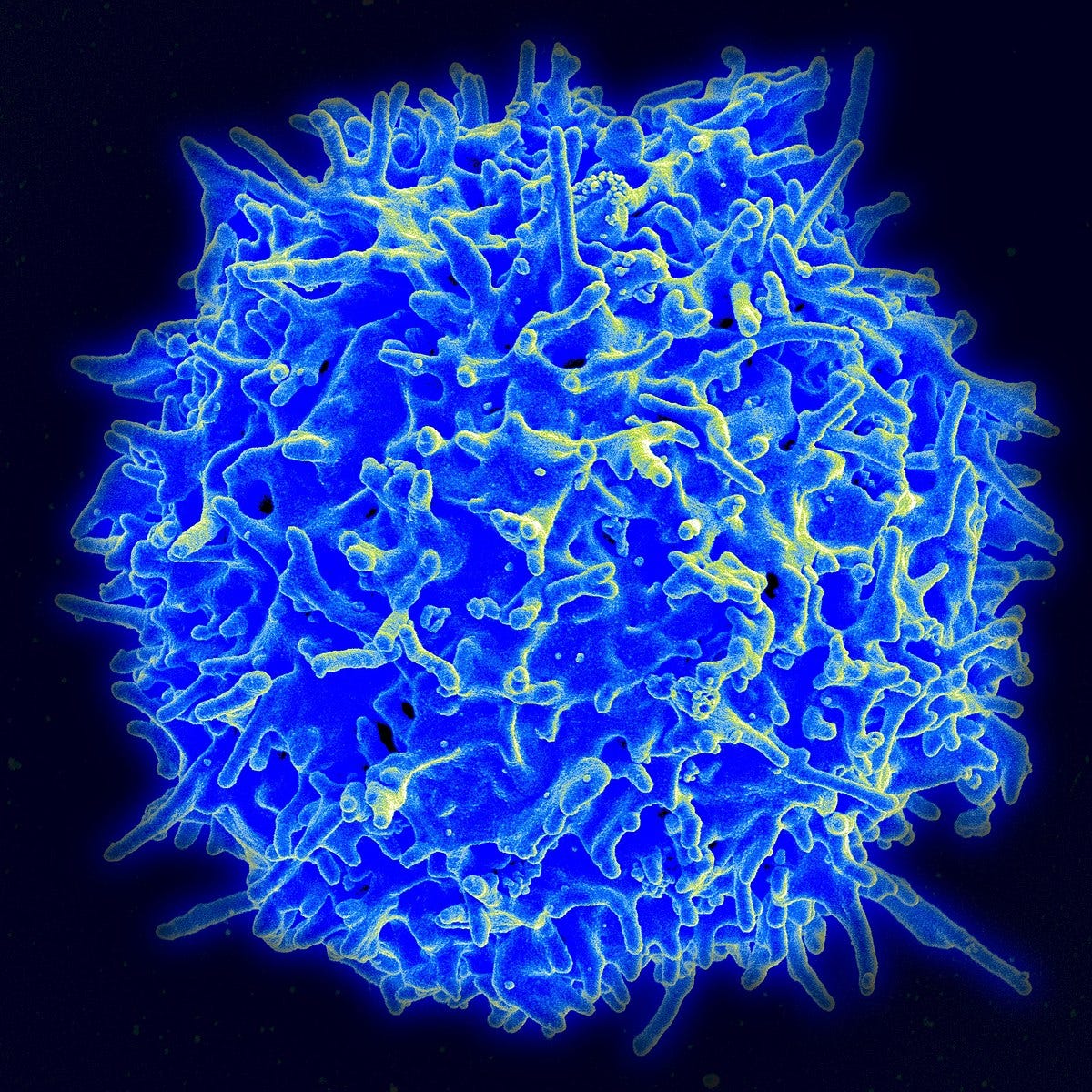
And here’s a macrophage.

Again, this is generally a good thing as it allows these cells to go to work and clean up debris and damage following injury. But, an unwanted consequence of this is damage to the nerve itself: for example, demyelination and axon degeneration. And, as Ji observed, immune cells also modulate pain in a manner similar to other non-neuronal cells, including the glia: they are capable of producing mediators that promote or dampen nociception in the neurons around them.
Interactions
By now, you might have noticed that neurons and microglia "talk to" each other. And more broadly, the sensory and immune systems also "talk to" each other. There is bidirectional signalling. To me, it is not that hard to get the idea of cells and molecules sensitizing a nociceptor. It is a bit less obvious that the nociceptor also takes part in the conversation. That is, nociceptors modulate inflammation. They too can express cytokines and trigger inflammatory cascades. They are not just wires but actively take part in the feed-forward mechanism of neuroinflammation.
Resolution
How does this all end? You might think that neuroinflammation kind of fizzles out. In fact, like classical inflammation, it is resolved by an active process. This process has another list of confusing molecules (cytokines like IL-4, IL-10, IL-13, TGFB and things called resolvins and endocannabinoids) but this time, they are anti-inflammatory. Microglia and nociceptors, too, release anti-inflammatory mediators.
Some researchers think that many chronic pain states might be a failure of this resolution mechanism.
To summarise…
So, let's bring this back to the people with radicular pain who participated in Albrecht's study. Now we have added some texture to this term "neuroinflammation" we can *tentatively* say something like this:
The study by Albrecht et al. shows that some (but not all) people with chronic radicular pain have ongoing, increased levels of neuroinflammation in their peripheral and central nervous systems. In these patients, peripheral nerve injury and ongoing nociception activated microglia in the spinal cord. These microglia released mediators which increased the excitability of nearby neurons, and reduced their inhibition. They also helped to trigger the activation of astrocytes, which have a similar but longer-lasting role. Other non-neuronal cells such as macrophages and t-lymphocytes may have migrated to these neuro-inflamed areas and crossed the blood-spinal cord barrier to infiltrate neurons themselves. All this became a feed-forward mechanism in which non-neuronal cells make neurons hyper-excitable, and hyper-excitable neurons also activate non-neuronal cells. Under normal conditions, an active process involving anti-inflammatory mediators should resolve neuroinflammation. But, for the participants in the study by Albrecht et al., it seems this has not happened, or is yet to happen.
That’s the end of the newsletter for this week! I had originally intended to talk more about the clinical significance of this, particularly when it comes to exercise. But I have already broken my promise to keep these newsletters short. I will leave that for a future edition…
I have added a few of the best papers I found to this google drive folder.
Please let me know if there are any errors or important additions. And do listen to the podcast with Dan, where he goes into more depth on his study and also delineates what we know and what we don’t know about neuroinflammation and pain…
Til next time!
Tom













Share this post